Health Systems face a high data entry and duplication burden for supporting clinical research studies. Having highly skilled people involved in data input limits your ability to participate in the scope of research that you can and should deliver. With the advent of new technologies for research independent sponsors are increasingly requesting the use of study specific technologies and processes for data capture and entry. Adapting to these heterogeneous requirements has made clinical research difficult to scale and still provide quality.
CLEHR helps to unburden your team from duplicate data entry by automatically populating EDC forms from EHR data. This improves the flow of information from health systems to sponsors offering a standard mechanism to support sponsor technical requests for efficiency. It adds the key capabilities to leverage existing investments into Health IT tools like FHIR interfaces and EHRs for research.
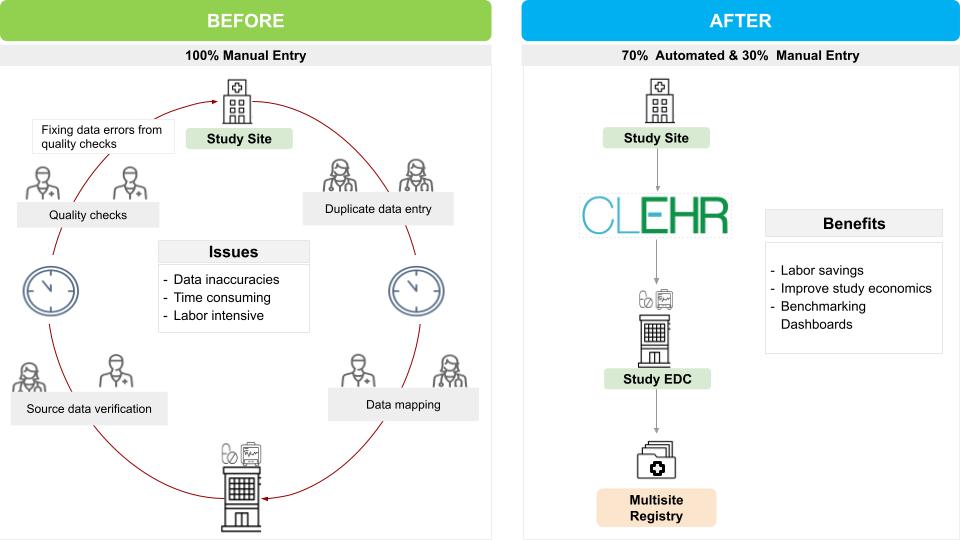
We understand that not all data entry for research studies can be extracted from EHR. That is why CLEHR is the solution to augment rather than replace your overall clinical research process. The focus is on avoiding 70% of manual work that can be automated. This provides significant quality advantages including automated testing and the volume of data shared.
Leverage your existing Health IT investments
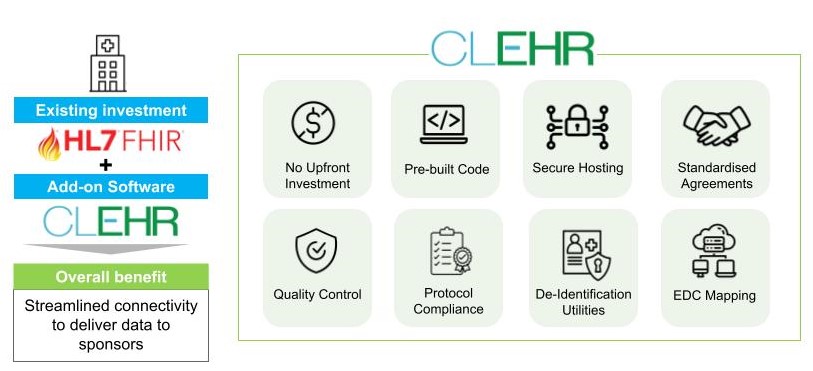
Use CLEHR to obtain high quality research outputs, improve engagement with sponsors, and perform multiple studies utilizing standard methods and infrastructure. Internal investigators and highly skilled research workers will be able to focus on performing quality research thanks to switching from manual data entry to the automated population of EDC forms.
 | Sponsors can collect data from multiple sites using the CLEHR solution. The Graticule team works with sponsors to establish study level data summaries. For participating studies, a dashboard displaying data on safety and results will be available to your research and quality teams. It will offer comparison statistics to other participating sites. You can use this data for benchmarking your processes against the best practices. |
CLEHR will enable you to
 |  |
| Control Costs | Initiate new studies faster |
| Graticule will assist you to expedite site setup through rapid contracting and one-time setup across multiple studies, saving you time and cost | You can use existing contracts and standard processes for conducting new studies across multiple sponsors |
Getting started with CLEHR
- Engage in the first study using CLEHR
- One-time infrastructure setup at health system with no direct costs
- Business Associate Agreement between Graticule and Health System
- Initiate with a study
- Expand and use as needed for multiple studies with the same sponsor or multiple sponsors
- Long Term CLEHR vision at your Health System
Benefits of partnering with Graticule
 |  |  |
| Execute more sponsored studies | Reduce the Complexity of studies | Prior experience of working with open standards |
| You can leverage the Graticule Research Network to gain access to more sponsored studies | Graticule will help you to establish standards for processes while working with existing sponsors to reduce the complexity of studies | Graticule brings in clinical informatics expertise including experience with open standards such as ODM and FHIR |
CLEHR Vision for Health Systems
Use CLEHR to improve your research partnerships
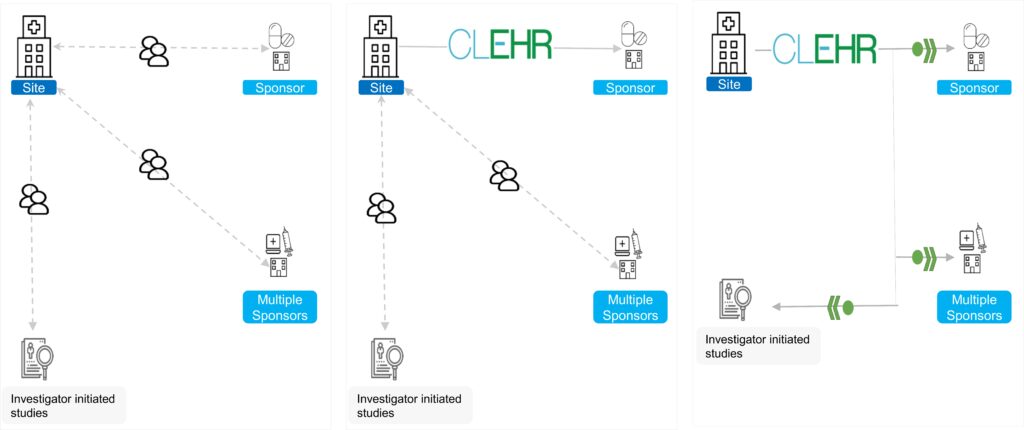
Key:

| 1.Before CLEHR | 2. Initial Implementation of CLEHR | 3. Enterprise Implementation of CLEHR |
| Most studies rely heavily on manual entry. Different technology provided by each sponsor further adds to the complexity. | CLEHR is implemented for one or multiple sponsors for multiple studies with common architecture. | CLEHR implemented for multiple studies with common infrastructure, security and quality control. Investigator Initiated Studies using automation provide higher efficiency processes in responding to Government or other NGO funded grants. |
CLEHR streamlines the data delivery process making it easier to collaborate with multiple sponsors at a lower cost. Internal users can benefit by using the same infrastructure for investigator initiated studies. CLEHR will facilitate high quality research at a lower cost by offering standard data access.
